Which Best Describes Partial Pressure in a Mixture of Gases
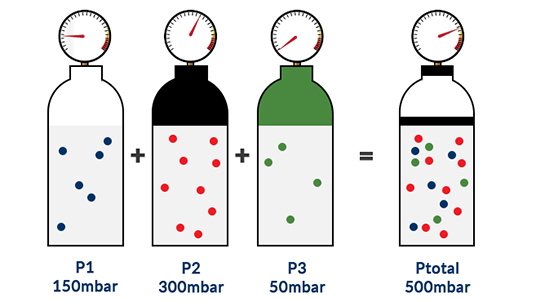
Pressure that is exerted by all the gases of a mixture on the container pressure that is exerted by one gas as if it occupied a container by itself half of the pressure that is exerted by the gases of a mixture on the container sum of the individual pressures that are exerted by two or more gases. P total P 1 P 2 P 3.
What Is Partial Pressure Of Oxygen And How Do You Calculate It
Which best defines partial pressure in a mixture of gases.
. In a mixture of hydrogen and helium gases the mole fraction of helium is 0750. Which of the following best describes Charless Law. O pressure that is exerted by all the gases of a mixture on the container o pressure that is exerted by one gas as if it occupied a container by itself o half of the pressure that is exerted by the gases of a mixture on the container sum of the individual pressures that are exerted by two or more gases.
The concentration 𝑛𝑉 of the mixture is found to be 0150 molL. The pressure of a gas is directly proportional to the temperature of the gas. The best definition is pressure that is exerted by one gas as if it occupied a container by itself.
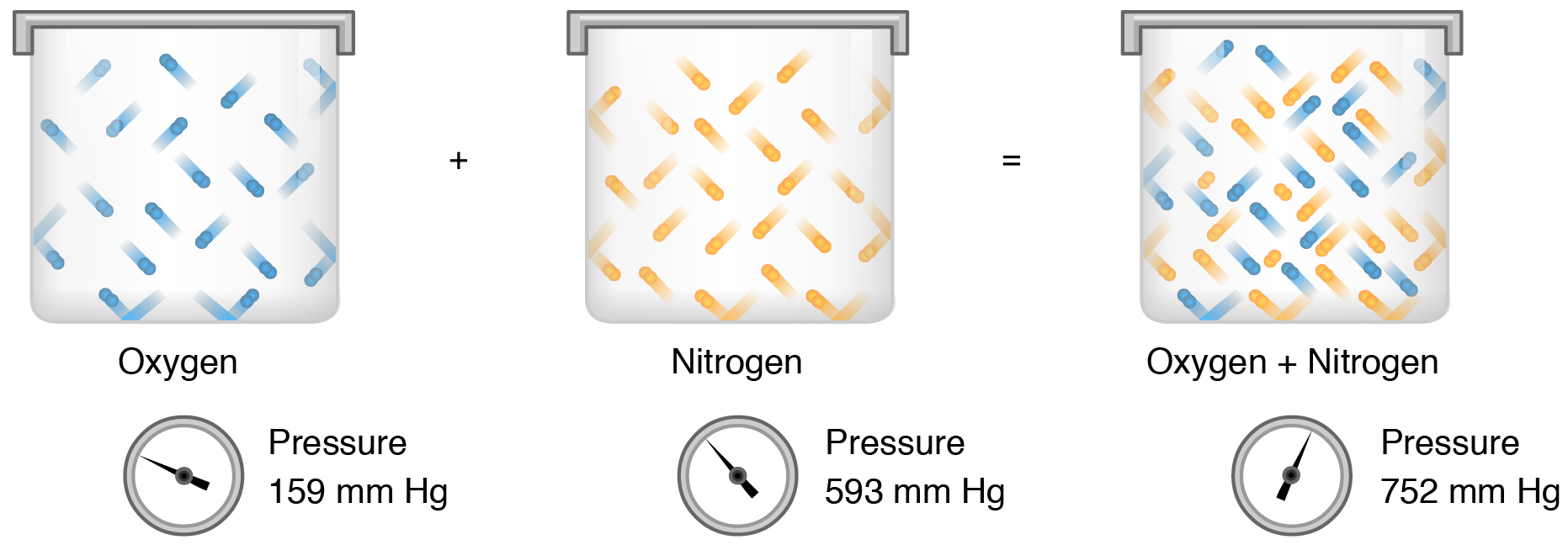
The best definition is pressure that is exerted by one gas as if it occupied a container by itself. The partial pressure of one gas in a mixture of gases contained in a given volume is the pressure that that one gas would exert if. If the partial pressure of hydrogen in the mixture is 75 torr what is the total pressure of the mixture.
A mixture of gases at a total pressure of 95 kPa contains N2 CO2 and O2. O O O O pressure that is exerted by all the gases of a mixture on the container pressure that is exerted by one gas as if it occupled a container by itself half of the pressure that is exerted by the gases of a mixture on the container sum of the individual pressures that are exerted by two or more gases. The partial pressure of one gas in a mixture of gases contained in a given volume is the pressure that that one gas would exert if.
According to Daltons law of partial pressures the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual gas and every gas is assumed to be an Ideal gas. Which best defines partial pressure in a mixture of gases. Which best defines partial pressure in a mixture of gases.
Since every gas has an independent behavior the ideal gas law is used to find. Assuming ideal behavior what is the partial pressure. Pressure that is exerted by all the gases of a mixture on the container essure that is exerted by one gas as if it occupied a container by itself half of the pressure that is exerted by the gases of a mixture on the container sum of the individual pressures that are exerted by two or more gases.
Which best defines partial pressure in a mixture of gases. The partial pressure of the CO2 is 24 kPa and the partial pressure of the N2 is 48 kPa. Where P 1 P 2 P 3 are the partial pressures of gas 1 gas 2 and gas 3.
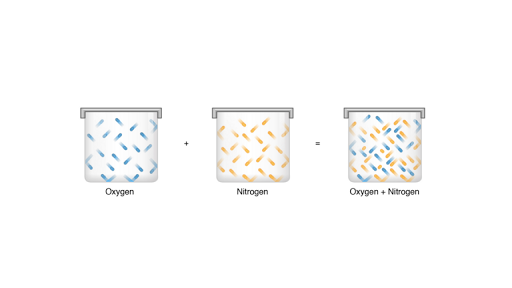
Dalton S Law Of Partial Pressure Article Khan Academy
No comments for "Which Best Describes Partial Pressure in a Mixture of Gases"
Post a Comment